Case Study: Using Real World Data to Estimate the Annual Incidence of a Rare Disease
This case study explores how a small pharmaceutical company utilized real-world evidence (RWE) to calculate the national incidence of a rare disease.

This case study explores how a small pharmaceutical company utilized real-world evidence (RWE) to calculate the national incidence of a rare disease.

At Magnolia Market Access, we see frailty as a key variable in evidence generation and access strategy. Ahead of ISPOR 2025, we sat down with Mike Murphy to discuss Magnolia’s latest research using administrative claims data to evaluate frailty indices.

The issue brief explores the PORTAL toolkit for Prescription Drug Affordability Boards (PDABs), highlighting key concerns and potential impacts on drug pricing, patient access, and policy implementation.
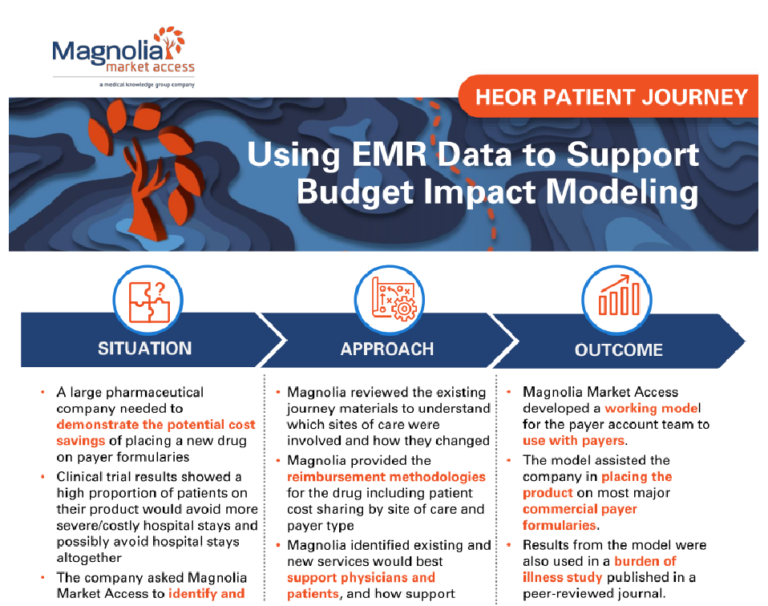
A large pharmaceutical company needed to
demonstrate the potential cost savings of placing a new drug on payer formularies.
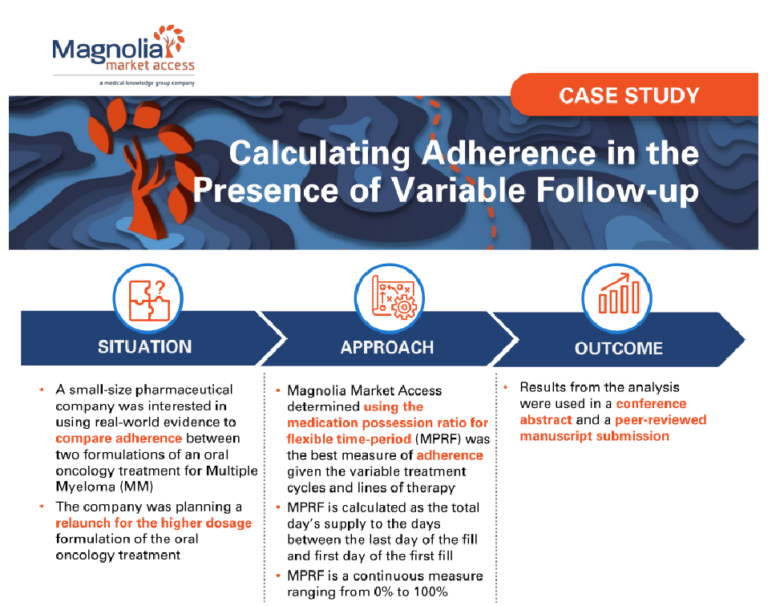
A small-size pharmaceutical company was interested in using real-world evidence to compare adherence between two formulations of an oral oncology treatment for Multiple Myeloma (MM).
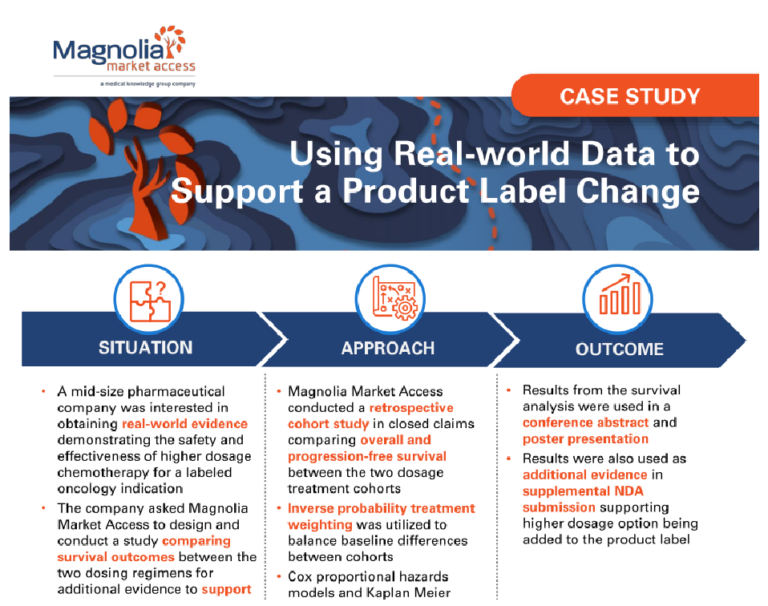
A mid-size pharmaceutical
company was interested in
obtaining real-world evidence
demonstrating the safety and
effectiveness of higher dosage
chemotherapy for a labeled
oncology indication.

AMCP Spring 2024 Platinum Poster and Presentation.

AMCP Spring 2024 Platinum Poster.
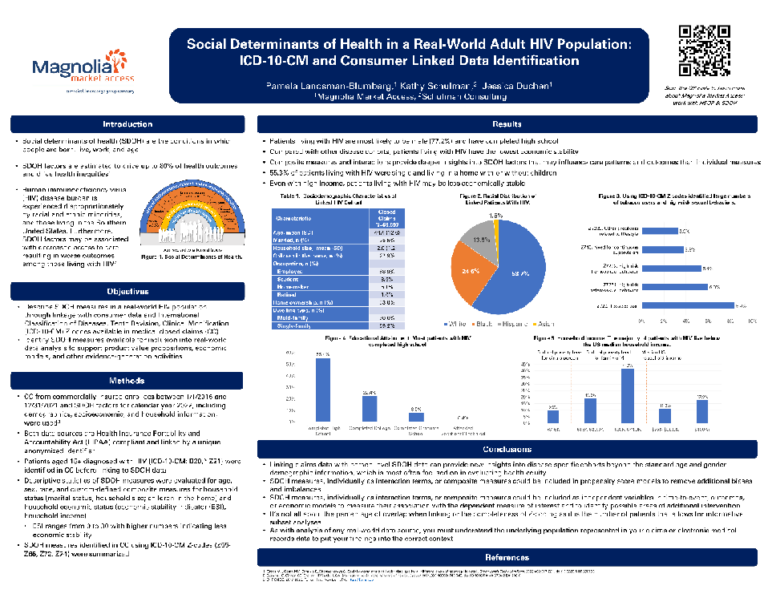

ISPOR Spring 2024.