Insights
We offer personalized insights and strategy, and spotlight data in a compelling way to shape policy, communicate value, secure reimbursement, and drive patient access.
-

Payer Research: A Strategic Driver of ROI Across the Lifecycle
Design research payers can use: focus on real-world outcomes, durability, and total cost of care; bring payer input in early and often; and pressure-test access levers pre-launch. Turn strong science into coverage, reimbursement, and measurable ROI.
-
Case Study: Using a Formulary Databook to Navigate 2025’s Medicare Part D Shifts
Find out how one manufacturer turned formulary complexity and policy uncertainty into strategic advantage using Magnolia Market Access’ Part D Formulary Databook.
-

Using Data-Driven Coverage Assessments to Unlock Medicare Part D Formulary Intelligence
As Medicare Part D plans adopt stricter utilization management, raise cost-sharing, and adjust tier placements, manufacturers face growing challenges in maintaining access. A data-driven formulary coverage assessment helps teams evaluate product positioning, benchmark competitors, and prepare for policy impacts — turning complexity into actionable strategy.
-

Navigating the Shifting Sands of Medicare Part D: Why Formulary Insights Are Critical for 2026 Planning
The Medicare Part D landscape is shifting fast. With IRA and H.R.1 driving formulary instability, manufacturers face rising deductibles, tighter utilization management, and new premium dynamics. Learn how Magnolia’s Part D Formulary Databook helps teams anticipate these changes and plan with confidence.
-

Case Study: Building a Gold-Standard Market Access Framework for a First-in-Class Therapy
See how Magnolia Market Access helped a manufacturer overcome coding gaps, payer unfamiliarity, and site readiness challenges to launch the first FDA-approved therapy in a rare disease — creating a replicable, gold-standard access model.
-

U.S. Physician Perspectives on International HTA and Drug Value Assessment
Survey of 100 U.S. physicians uncovers key insights on how international HTA frameworks—especially Germany’s—undervalue chronic disease therapies. Explore the full infographic.
-

Case Study: Evidence Strategy & Value Messaging Support for an Approved Oncology Product
A small medical device company engaged Magnolia Market Access to strengthen its evidence strategy and value messaging for an innovative oncology device. Despite strong clinical results, the company needed to address evidence gaps and ensure its value story resonated with payers and providers.
-
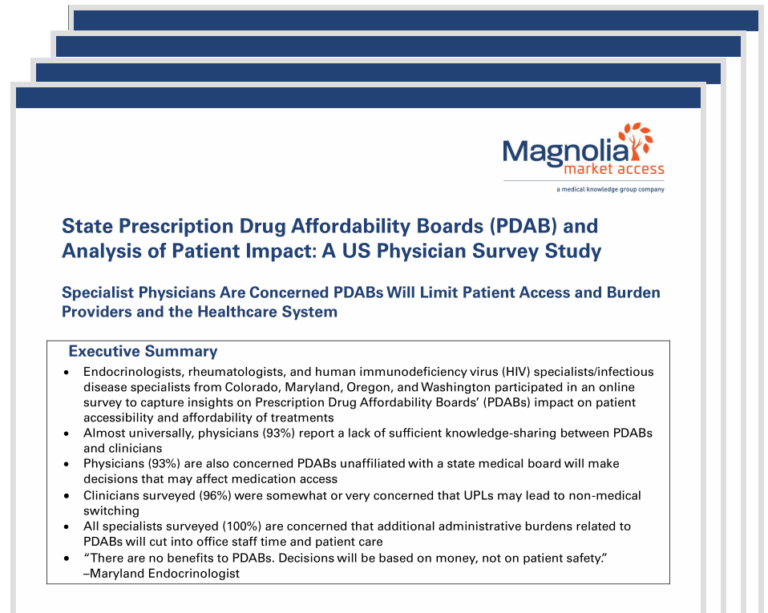
Specialist Physicians’ Perspectives on State PDABs: Access, Affordability, and Administrative Burden
As states expand PDAB authority to set Upper Payment Limits (UPLs) on high-cost therapies, Magnolia Market Access surveyed physicians in Colorado, Maryland, Oregon, and Washington to understand how these policies may affect prescribing decisions, patient care, and clinical operations.
-
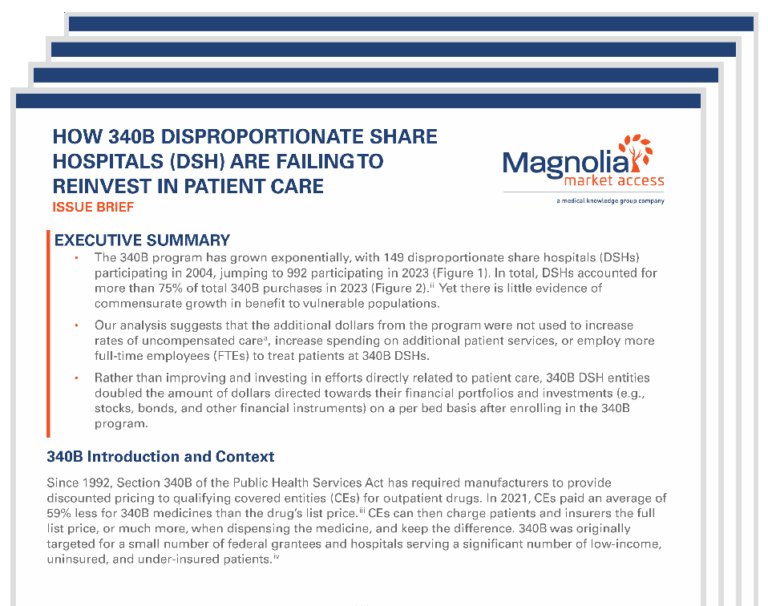
How 340B Disproportionate Share Hospitals (DSH) are Failing to Reinvest in Patient Care
This issue brief provides insights into how 340B savings are being redirected by covered entities—particularly DSH hospitals—toward financial investments instead of patient care, underscoring the urgency of policy changes to realign the program with its mission.
-

Policy is the New Payer: Why Access Strategy Must Now Navigate HHS, MFN, and Tariffs Together
In the June 2025 Navigating Market Access with Magnolia webinar, experts from across the market access ecosystem came together to examine the forces reshaping how manufacturers plan for pricing, reimbursement, and access.
-

Unlocking the Value of RWE in Drug Pricing Decisions
In this featured presentation from the 2025 Evidence 360 Summit, Magnolia Market Access’s Beni Turner explores how RWE can be strategically integrated across the product lifecycle to drive more effective pricing and access outcomes.
-

Case Study: Using Real World Data to Estimate the Annual Incidence of a Rare Disease
This case study explores how a small pharmaceutical company utilized real-world evidence (RWE) to calculate the national incidence of a rare disease.