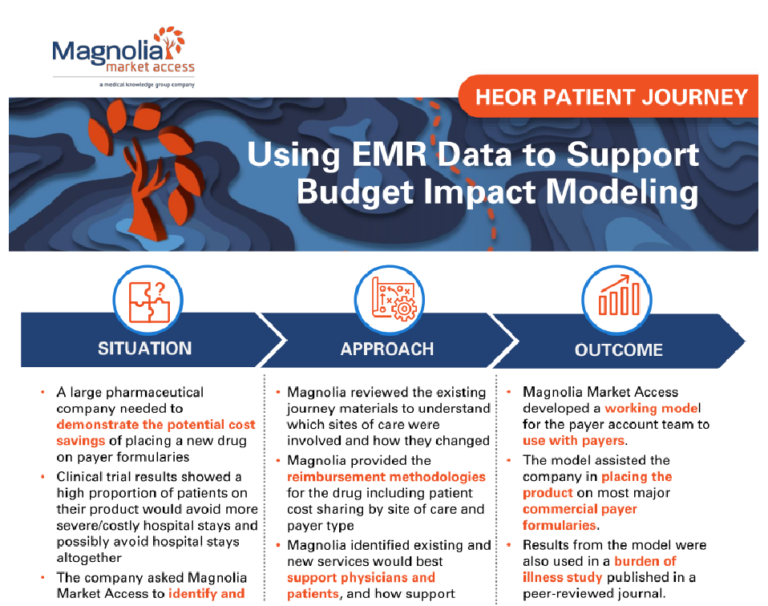
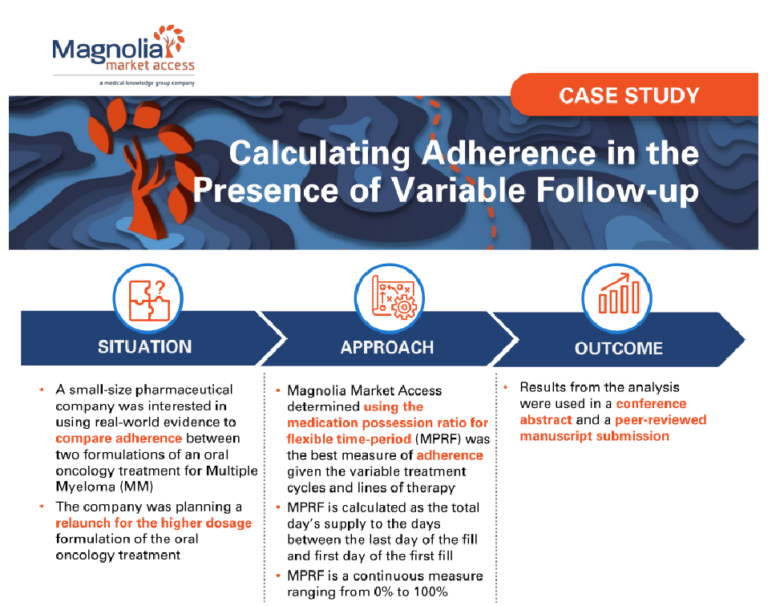
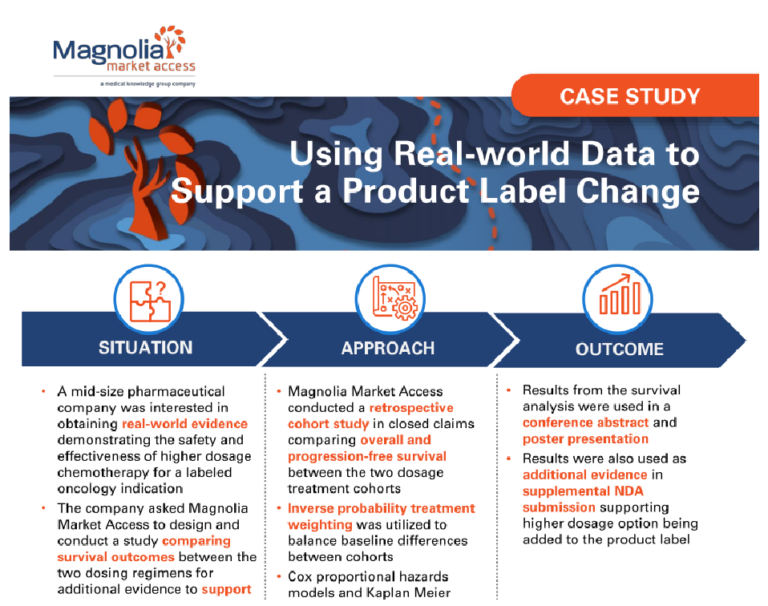
Building a Comprehensive Go-To-Market Strategy Through Stakeholder Ecosystem Mapping
A mid-size client was preparing for a product launch and needed assistance facilitating a successful go-to-market strategy. The company sought to identify and understand the entire stakeholder landscape and create short- and long-term stakeholder engagement strategies to meet the needs of their entire organization.