Case Study: Launch Planning and Readiness
How program management and strategic alignment prepared a biopharma company for NDA submission and launch.

How program management and strategic alignment prepared a biopharma company for NDA submission and launch.

How payer research helped refine value messaging, pricing strategy, and evidence plans.

How a pharmaceutical company built a compliant, scalable 340B contract pharmacy program.

In the June 2025 Navigating Market Access with Magnolia webinar, experts from across the market access ecosystem came together to examine the forces reshaping how manufacturers plan for pricing, reimbursement, and access.

A mid-size client was preparing for a product launch and needed assistance facilitating a successful go-to-market strategy. The company sought to identify and understand the entire stakeholder landscape and create short- and long-term stakeholder engagement strategies to meet the needs of their entire organization.
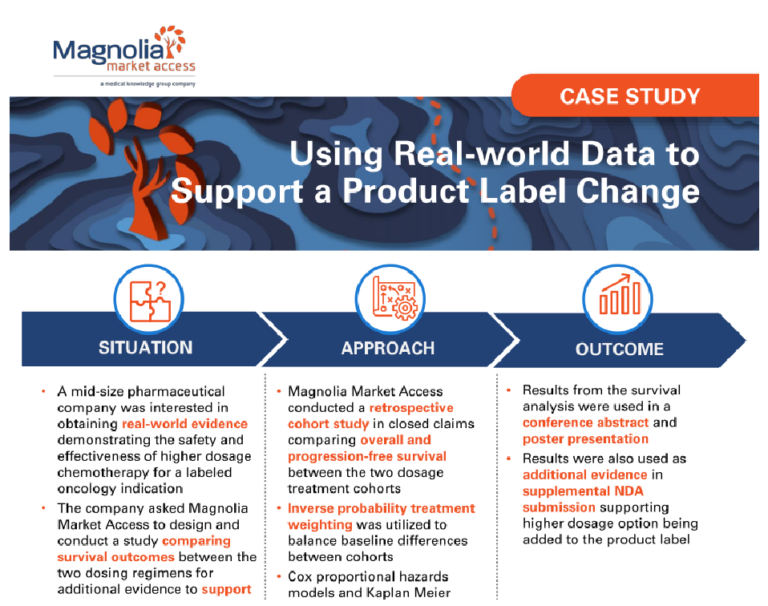
A mid-size pharmaceutical
company was interested in
obtaining real-world evidence
demonstrating the safety and
effectiveness of higher dosage
chemotherapy for a labeled
oncology indication.

Navigating Market Access with Magnolia Summer 2024 Presentation.

Magnolia was asked to assess which type of cell therapies and disease states offered the greatest potential for development, and also wanted to further understand the benefits of creating an autologous or allogeneic product.

Magnolia was engaged as a payer agency of record for a mental health digital therapeutic. Although the company had experience launching drugs in the U.S. market, the novelty of the digital therapeutic space was challenging, and the company did not have experience in mental health, nor a comprehensive launch plan in place across channels.

A biopharma company engaged Magnolia to develop a value story for an orphan-designated treatment for an auto-immune disorder that was in Phase III development. The company’s product had a novel mechanism of action in comparison to the existing agents in development and would be the first new agent for the disease in years.